Virus replication depends on interactions between viral and host cell proteins to overcome cellular defenses. The SV40 large T antigen (T-Ag) oncoprotein binds to numerous cellular proteins to enable efficient viral replication. Although the C-terminal region of T-Ag has no evident enzymatic activity, mutations in the C-terminus of T-Ag disable the ability of SV40 to replicate and undergo lytic infection in restrictive cell types but not others.
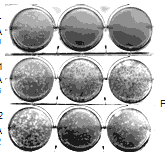 Our high-throughput proteome-scale interactome screens found that the uncharacterized host protein FAM111A binds specifically to the C-terminal region of T-Ag. Following up on this intriguing observation, we determined that this interaction is required for efficient viral replication and sustained viral gene expression in restrictive cell types. Depletion of FAM111A significantly increases viral gene expression and enables lytic infection by SV40 host range mutants. Fine-mapping of the interaction domains identified a C-terminal trypsin-like peptidase domain in FAM111A that is is required for T-Ag binding. The identification of FAM111A as a host range restriction factor (Fine et al, PLoS Pathog, 2012 in press) was only possible through the genome-scale screens of viral-host perturbations undertaken in the course of this CEGS.
Our high-throughput proteome-scale interactome screens found that the uncharacterized host protein FAM111A binds specifically to the C-terminal region of T-Ag. Following up on this intriguing observation, we determined that this interaction is required for efficient viral replication and sustained viral gene expression in restrictive cell types. Depletion of FAM111A significantly increases viral gene expression and enables lytic infection by SV40 host range mutants. Fine-mapping of the interaction domains identified a C-terminal trypsin-like peptidase domain in FAM111A that is is required for T-Ag binding. The identification of FAM111A as a host range restriction factor (Fine et al, PLoS Pathog, 2012 in press) was only possible through the genome-scale screens of viral-host perturbations undertaken in the course of this CEGS.